This is a joint blog post by Leigh Purvis and Debra B. Whitman, Ph.D.
Hepatitis C is a serious liver disease that results from infection with the hepatitis C virus. It currently affects more than three million Americans, many of whom do not know that they are infected. Left untreated, it can lead to liver damage, cirrhosis, liver failure, or liver cancer.
Until recently, hepatitis C treatments only cured about half of patients and often had undesirable side effects. Fortunately, a new drug called Sovaldi entered the market in late 2013 that had a higher cure rate, a shorter duration of treatment, and fewer side effects than previous treatments. However, this remarkable advance came with an equally remarkable price: $1,000 per pill, or $84,000 for a typical course of treatment.
Why so expensive?
Gilead, the drug company that makes Sovaldi, argues that drug's high price pays for itself by avoiding future complications like liver transplants. However, experts have thus far concluded that projected savings would not offset the cost.
In addition, sales of Sovaldi are increasing at a record-breaking pace, with analysts expecting U.S. sales of $10 billion in 2014. Gilead bought the company that originally developed Sovaldi for $11 billion, and its entire research and development budget was just over $2 billion in 2013. Thus, Gilead will likely recoup its investment in less than two years based on its U.S. sales alone, raising questions of whether Sovaldi's high price is really just profiteering.
It is also noteworthy that Sovaldi's price is considerably lower outside of the U.S. While the U.S. government continues to decline to regulate drug prices, many other countries have been able to obtain Sovaldi for far less than $84,000.
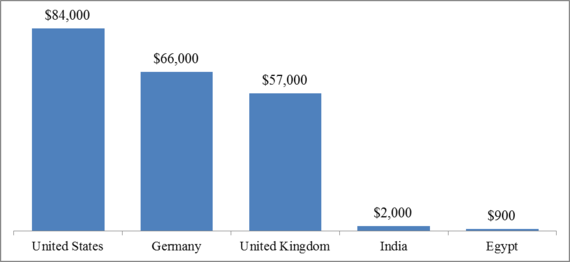
Source: B. Berkrot and D. Beasley, "U.S. lawmakers want Gilead to explain Sovaldi's hefty price," Reuters, March 21, 2014.
Insurers' response to Sovaldi
Many payers and pharmacy benefit managers have begun to push back against Sovaldi's price, with some threatening to stop using the drug once a rival medicine is approved in the United States. State Medicaid directors have also raised concerns, saying that taxpayers will have to shoulder much of Sovaldi's costs since many hepatitis C patients get their health care from the government.
Some insurers, including a number of state Medicaid programs and the Department of Veterans Affairs, have decided to limit use of Sovaldi to patients with more advanced disease, saying others can wait without risk because the disease progresses slowly. This approach has also been advocated by an expert panel.
Larger questions loom
The debate surrounding the introduction of Sovaldi is part of a much larger issue: escalating specialty drug prices that are widely viewed as unsustainable. Specialty drugs now account for 28 percent of total drug spending in the U.S. even though they make up less than 1 percent of all prescriptions.
The costs associated with specialty drugs impact everyone in the health care system, regardless of whether they are taking one themselves, through increased premiums and cost sharing. Patients who are prescribed a specialty drug also face the possibility of high out-of-pocket costs that could push them to forgo care, particularly if their insurer requires them to pay a percentage of their prescription drug cost instead of a flat copayment.
There are growing signs that drug pricing is approaching a breaking point. For example, the American Society of Clinical Oncologists recently announced that it was initiating a cost-benefit analysis for existing cancer drugs. Similarly, more than one hundred cancer specialists co-authored an article that criticized the high prices of cancer drugs. And, in one highly publicized incident, physicians at Memorial Sloan-Kettering Cancer Center refused to use an expensive new cancer drug, saying it was no better than older, less expensive drugs.
In the meantime, Gilead is working on a combination hepatitis C drug that is expected to be approved by the FDA later this year. Rumored price? $140,000.