There are a few moments in life when we have the clairvoyance to see into the future with confidence that the vision is real. Such was the case for health care and medicine on December 19, 2013, with the publication of the editorial authored by Francis Collins, Director of the National Institutes of Health, and Margaret Hamburg, Commissioner of the Food and Drug Administration, in The New England Journal of Medicine. This editorial, titled "First FDA Authorization for Next-Generation Sequencer," is a view into the future of health care and the impact it will have on medicine.
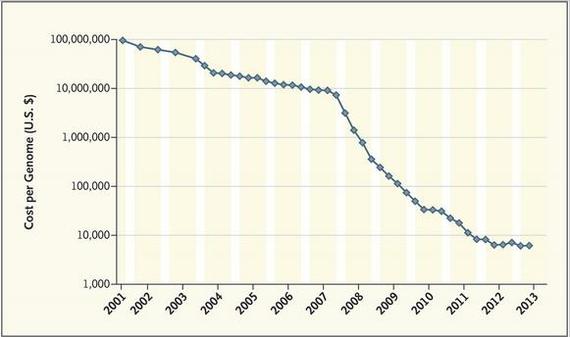
Drs. Collins and Hamburg state that it has been 60 years since Watson and Crick described the structure of DNA and 10 years since the complete sequencing of the human genome was achieved, a milestone that was announced on the lawn of the White House Rose Garden. The cost of sequencing the first human genome approached a billion dollars and took almost ten years to complete, but today, due to massive technological advances, the cost is dropping to less than $5,000 and can be done in less than 24 hours (see figure).
The article announces that the FDA has just approved the first high throughput (next-generation) DNA sequencing machine for medical diagnostic work. This will allow for the acceleration of the application of DNA sequencing to many fields of medicine. Two of the most immediate applications for this technology are in cancer diagnostics and therapeutics and in analysis of a patient's potential response to specific medications before treatment.
Traditionally cancer therapies have been based upon the diagnosis of the tumor type, such as adenocarcinoma or neuroblastoma, as well as the tissue type and the staging of the tumor (the progression of the cancer). As Collins and Hamburg point out in their article, however: "...recent work from the Cancer Genome Atlas demonstrates that the tissue of origin of a particular cancer [i.e., its traditional diagnosis] may be much less relevant to prognosis and response to therapy than the array of causative mutations" [i.e., that which can only be understood from genomic analysis of the tumor itself]. This has opened up an entirely different approach to cancer therapy that is based upon the unique genetic characteristics of the patient's tumor and how it responds to various therapeutic agents, as contrasted to treating all types of cancer within a certain diagnostic class based upon tissue type of the tumor. This approach to cancer diagnosis and therapy represents the concept of personalized medicine. The focus is less on what the disease is called and more on the unique genomic characteristics of the individual patient's disease.
By the same token, this technology will be applied to determining the unique genetic responses a person has to specific therapies or drugs based upon their genomic uniqueness. In a seminal publication in the Journal of the American Medical Association in 1998, Lazarou , Pomeranz and Corey reported that the overall incidence of serious adverse drug reactions in hospitalized patients was 6.7 percent and of fatal adverse drug reactions was 0.32 percent. They estimated that in 1994, an overall 2,216,000 hospitalized patients had serious adverse drug reactions and 106,000 had fatal adverse drug reactions, making these reactions between the fourth and sixth leading cause of death in the United States (JAMA 1998; 279: 1200-1205). Now and in the future, genomic information provided by sequencing will be able to identify the right drug at the right dose for each patient, thereby potentially preventing tens of thousands of adverse drug reactions and preventable deaths. This technology will alter prescribing patterns and the way that medicine is practiced, and move therapeutics to individualized treatment away from "medicine for the average person."
Genome sequencing needs to be done only once for each individual. The data can be stored electronically in a digital medical record for the rest of one's life. This information can be accessed and queried whenever new questions arise concerning a medical decision or issue, or when new information on the relationship of specific genetic characteristics to health is made available. In a sense, the sequencing of the whole genome of an individual is the "mother of all laboratory tests" in that encoded in the person's genomic sequence is information about all subsequent health questions. The approval by the FDA of the Illumina MiSeqDx sequencer for use in medical diagnostic applications represents a major step forward in integrating full genomic data into the future decision-making in health care and medicine. It has been speculated that with the rapidly declining cost of a full genome sequencing that we are on the threshold of the "$1,000 genome analysis." When this occurs -- and it will happen within the next few years -- the cost of this test will be competitive with many traditional diagnostic tests and be widely incorporated into patient care and reimbursed for by insurance companies.
The adoption of this technology will create a sea change in medicine. It will require physicians to know more about genetics as it relates to diagnosis and therapy. As Collins and Hamburg state in their article:
Doctors and other health care professionals will need support in interpreting genomic data and their meaning for individual patients. Patients will want to be able to talk about their genetic information with their doctor. With the right information and support, patients will be able to participate alongside their doctors in making more informed decisions.
We can now start to see the future of health care and medicine more clearly. We are moving from a medicine for the average to a medicine for the individual. We are starting to recognize that lifestyle, diet and environmental factors influence how our genes are expressed into our health and disease patterns. In 2013, Dr. Deanna Minich and I authored the article "Personalized Lifestyle Medicine: Relevance for Nutrition and Lifestyle Recommendations," in which we discuss how access to genomic information, along with information about the patient's lifestyle, diet, medications and environmental exposures, will frame a new approach to health care and medicine in the prevention and treatment of chronic diseases (Scientific World Journal 2013; 129841).
The acceleration in the development of genomic technology and its application to health care and medicine is a force that is rapidly changing medical education, training, standards of care, health care delivery and reimbursement. What were considered "rules of the road" a few years ago, related to how genomic information would apply to health care, is undergoing revolutionary changes. It doesn't take clairvoyance to see the future. The future will be the incorporation of personalized medicine into health care. And it cannot happen soon enough. The approach of "medicine for the average" that has been applied to the management of the global epidemic of chronic disease has not been successful in reducing the rising burden of preventable disease. We need a new approach based upon individual needs and personalization. As was stated by Bayer, Fairchild, Hopper and Nathanson in a recent Science magazine article titled "Confronting the Sorry State of U.S. Health," the United States ranks last among peer nations in health status and compares unfavorably to 17 peer countries at almost every stage of life course including heart disease, diabetes, cancer, chronic pulmonary disease, autoimmune diseases, and dementia (Science 2013; 341: 962-63). The article closes with the call to action of "mobilization of an unprecedented kind is now necessary in the United States. It requires a campaign to remove the public veil of ignorance about the evidence."
I believe that the announcement by the FDA of the approval of the genome sequencing technology for medical applications is symbolic for the future development of personalized health care. The next step is to recognize the importance of a patient-centered medicine that integrates genomic information with lifestyle, diet, environmental and social understanding to form the more cost effective system that lies over the immediate health care horizon.
Dr. Jeffrey Bland is the author of the upcoming book The Disease Delusion, scheduled for release by HarperWave, a division of HarperCollins Publishers, in Spring 2014.