"Mars Limb" Image Copyright 2015 by Marc Dantonio
Here and there on the net in forums and on Facebook sites there are numerous posts that discuss the case for liquid water on Mars. The NASA rover photos are often shown in false color which can make some areas look like they are wet or contain standing water. But what is REALLY going on with Mars? Can standing water exist? Has it ever existed? This article discusses a little bit of the science of Mars and its past, and looks at conditions today which are markedly different from early Mars.
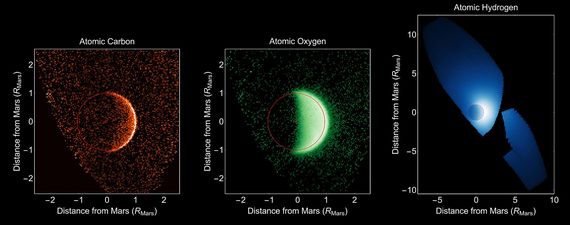
Early in Mars' history, its atmosphere was much thicker than it was today. In those earlier times, water likely existed in standing pools if not global oceans depending on the hypothesis. Mars' atmosphere though was thinned over time due to a number of different factors. For one thing, the molten core cooled to near solid and the magnetic field thus diminished leaving Mars without a fully encompassing and protective magnetic field. This allowed the high energy radiation from the sun to strike the atmosphere directly which destroyed molecules of water, carbon dioxide and oxygen for instance. The resulting atoms were lost to space, a process that continues to this day. NASA's Maven mission clearly shows carbon and oxygen bleeding off of Mars slowly year by year. ( see Fig 1 below).
Fig 1:NASA's Maven spacecraft data showing how carbon, oxygen, and hydrogen are 'bleeding' off of Mars to this day. Public Domain Maven Image courtesy of NASA.
Another way that early Mars could have lost atmosphere was during massive impacts with meteors and asteroids. Mars' gravity is only 0.38 times that of Earth. So an impact of a large asteroid or meteor would have more than enough energy to not just blast a crater into the surface, but to blow large volumes of atmosphere into space as well. There are impact basins on Mars that indicate that very large objects have struck the planet in the past. It is quite conceivable that these were responsible for a significant amount of atmospheric loss. As the Martian atmosphere thinned, the pressure and temperature dropped on the surface. Over time, standing water boiled away, froze into solid ice, or reacted chemically with the soil. The processes that shape the Mars we see today occurred billions of years ago.
But lets look at water for a moment and discuss some of its properties because this will help answer the question of whether water can pool on today's Mars. Everyone knows that when you heat water it boils and that it will eventually boil away turning to water vapor. But not everyone knows that boiling isnt related only to temperature. Atmospheric PRESSURE is very important as well. A higher pressure means that higher temperatures are required to boil water and lower pressures mean lower temperatures are required to do the same.
At Earth's atmospheric pressure which is pressing down on us all at an astounding 14.7 pounds per square inch, water needs to be heated to 100°C (212°F) in order to begin the transformation to water vapor that we all know as boiling. But as the pressure drops, so too does the temperature required to make water boil. Near pressures of 0 pounds per square inch, water will boil at room temperature. But similarly if you drop the temperature down towards absolute zero while decreasing the pressure, then water will not boil but will freeze. So pressure and temperature work together to change the state of water.
But lets look at a curiosity of water for a moment. Water can exist as we know in three states, solid, liquid, and gas. You can imagine then that at a certain temperature and pressure, water may be at the borderline of being solid, liquid OR gas. This point where the conditions exist for all three states to exist at the same time is called the Triple Point. In other words, water is just as likely to be frozen, gaseous, or liquid at this triple point condition.
On Mars the temperature required at the triple point of water is just about 0°C (32°F) and the pressure required at the Martian triple point is 0.088 pounds per square inch. Oddly enough, on Mars the average surface pressure is 0.087 pounds per square inch. So for the remotest possibility of liquid standing water to exist on Mars we would have to look to the deepest canyons where the pressure was higher and perhaps at least that required by the Triple Point condition.
Finding the pressure to be about right isnt a problem. There are those low elevation areas on Mars where the pressure meets or exceeds the triple point pressure of water of 0.088 pounds per square inch. The problem comes when we try to find the right temperature of 0°C on the other hand. It turns out that this would be a long shot at best. Mars averages temperatures that are nearly always well below 0°C. So in practice the minimum required condition that needs to be met, that of the Triple Point of water, can only be met under the briefest of circumstances on the red planet.
But there is a caveat. Although Mars has plenty of water ice locked up under the surface, reaching the Triple Point however briefly doesnt mean water is suddenly melted from the ice. It means that liquid water could exist as ice, vapor or liquid but doesn't mean ice will necessarily melt nor that water vapor may necessarily condense into water. The real creation of copious flowing water would require a specific event locally that might spark a sudden melt of liquid water. Such an event could be an avalanche that creates a local pressure wave, or an impact by a meteor or some such event.
We HAVE seen strange outflow patterns from inside the rims of craters where it appears water suddenly gushed out, created an outflow gully and then was gone. There are theories as to why that may occur and those theories require the Triple Point being reached at the very least for a brief time. But any water liberated on Mars can never be found in standing pools or flowing rivers.
Thanks for reading! See you next time.