The collagen meniscus implant (CMI) is back on the US market, and it has the potential to change the way knees are treated. Here is why:
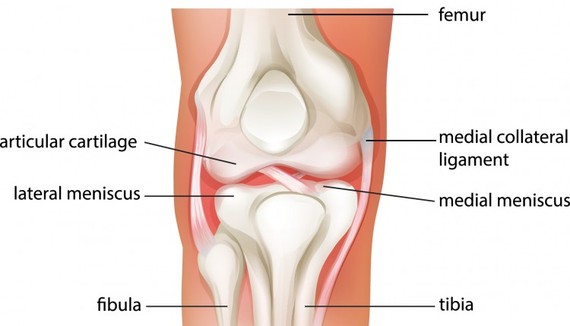
The meniscus is the key fibrous shock absorber in the knee joint.[1] It is torn and partially removed, according to the National Center for Health Statistics, more than a million times in the United States each year. Unfortunately, surgeons only repair less than 10% percent of all the torn meniscus cartilages they see. Why? Because it is difficult to repair, and has a variable history of healing.[2] The side effects of losing the meniscus--pain and arthritis--usually don't present for years.[3] For that reason, the quicker and less expensive meniscectomy (excision of the meniscus) is performed.
The meniscus gets torn by being pinched between the femur and the tibia. This can happen during sports, or through any bending or twisting motion--sometimes just getting out of a car is enough.[4] Often a pop or sudden pain occurs, sometimes with swelling. The torn tissue acts like a "windshield wiper" in the knee joint, mechanically causing pain and wearing away the opposing articular cartilage surface. The cells of the torn, unstable meniscus also release factors that speed up the onset of arthritis.[5]
The CMI -- which I designed in 1986 -- acts in two ways. First, it is a physical scaffold upon which new meniscus tissue can grow. Second, it contains growth factors and stem cell holders able to actually stimulate regrowth in areas previously not repairable. [6,7] The implant was initially approved in the US for wide clinical use--but because of a political conflict between the manufacturer and the FDA, it was taken off the market in 2010. Now it's back, having being used successfully in more than 6,000 cases worldwide.
The promise of the CMI is that it opens up a whole new field of meniscus reconstruction. Tissues once deemed irreparable can now be regrown. Missing segments of meniscus cartilage can be selectively replaced by sewing in the scaffold and inducing remodeling.[7] Patients with joint pain caused by loss of this natural shock absorber can now be relieved of suffering by restoring their meniscus.
While complete meniscus replacement (using a donor meniscus) has proven successful for more than two decades, most people only need a part of the meniscus restored. The CMI was designed to do just that. Potentially hundreds of thousands of patients whose knees were doomed to develop arthritis as a result of meniscus removal may now have their knees reconstructed. The demon of arthritis may not yet be slayed--but it has certainly been weakened.
______________________
1. Rath, Ehud, and John C. Richmond. "The menisci: basic science and advances in treatment." British journal of sports medicine 34, no. 4 (2000): 252-257.
2. Thorlund, J. B., C. B. Juhl, E. M. Roos, and L. S. Lohmander. "Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms." bmj 350 (2015): h2747.
3. Roos, H., Laurén, M., Adalberth, T., Roos, E. M., Jonsson, K., & Lohmander, L. S. (1998). Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty‐one years, compared with matched controls. Arthritis & Rheumatism, 41(4), 687-693.
4. Poulsen, Matthew R., and Darren L. Johnson. "Meniscal injuries in the young, athletically active patient." Physician and Sportsmedicine 39, no. 1 (2011): 123-130.
5. Driban, Jeffrey B., Robert J. Ward, Charles B. Eaton, Grace H. Lo, Lori Lyn Price, Bing Lu, and Timothy E. McAlindon. "Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: Data from the osteoarthritis initiative." Clinical Anatomy 28, no. 6 (2015): 792-799.
6. McCrum, Christopher L., and C. Thomas Vangsness. "Postmeniscectomy Meniscus Growth With Stem Cells: Where Are We Now?." Sports medicine and arthroscopy review 23, no. 3 (2015): 139-142.
7. Steadman, J. Richard, and William G. Rodkey. "Tissue-engineered collagen meniscus implants: 5-to 6-year feasibility study results." Arthroscopy: The Journal of Arthroscopic & Related Surgery 21, no. 5 (2005): 515-525.